The MULTIPLI Program takes place in a larger and more ambitious national initiative: The French Genomic Medicine 2025 Plan. This plan aims to construct a medical and industrial system to introduce genomic medicine into the care pathway and develop a national framework in this matter.
About the France Genomic Medicine 2025 Plan
The France Genomic Medicine 2025 Plan fulfils the commission entrusted by Prime Minister to the French National Alliance for Life Sciences and Health (Aviesan) in April 2015, to investigate establishing access to genetic diagnosis in France.
Many issues are raised by genomic medicine, in terms of:
- Public health: With the spread of ultra-high-throughput sequencing, significant proportions of patients will ultimately benefit from routine genomic investigation, not only patients with rare diseases and cancer but also those with certain common diseases. Higher resolution diagnosis will enhance care with shorter time frames as well as more effective therapeutic strategies and fewer adverse reactions.
- Socioeconomics: Reduced health care expenditure is likely to be substantial by virtue of various factors: a reduced number of expensive but spurious diagnostic procedures; quicker test turnover; avoidance of ineffective, potentially dangerous drug treatments and the need to treat adverse reactions; earlier treatment; and more effective treatment leading to increased life expectancy. In parallel, a new, innovative industrial framework will be constructed, a source of economic development and employment.
- Technology: The integration of ultra-high-throughput sequencing in the medical practice will create a dynamics in the capacity to acquire, store, distribute, match and interpret big data from diverse sources, with the development of servers, cores and intensive calculation methods, and software for data mining and modelling.
These major issues explain the strong international competition in the field of genomic medicine, and the need for France to reach a high level in this matter.
The France Genomic Medicine 2025 (FGM 2025) Plan exploits particularities of the French health-care system which integrate patient care, training, and research with the development of broad-scope actions strongly supportive of this approach (governmental plans in the fields of cancer, neurodegenerative and rare diseases, and co-definition by public and private partners of research strategies).
The ethical dimension is an integral part of the Plan. It has to provide answers to the numerous questions being asked by citizens and patient support groups on consent in access to and exploitation of health data, the anonymization of data vis-à-vis third parties, how secondary discoveries and unwanted incidents are to be handled.
The Genomic Medicine 2025 Plan aims to:
- Position France among the countries leading the way in personalized and precision medicine with export of the expertise derived from the French medical and industrial genomic medicine system.
- Prepare for integration of genomic medicine into the care pathway and the management of common diseases. This means setting up a generic care pathway with access for all patients with cancer, a rare disease or a common disease by 2025, with genomic medicine available to all affected patients in the country.

- Set up a national genomic medicine framework capable of driving scientific and technological innovation, industrial capitalization and economic growth.
The Plan articulates around three main targets with a series of 14 measures to:
Establish instruments for a genomic care pathway (Target 1):
- Measure 1: Construct French capacity for ultra-high-throughput sequencing to meet the defined targets
- Measure 2: Develop the tools required to process and exploit the volume of data that will be generated with creation of a central analysis service (CAD, collecteur analyseur de données)
- Measure 3: Foster the integration and exploitation of patient data into the care pathway
Ensure operational deployment and expansion of the system in a safe and ethical technical framework (Target 2):
- Measure 4: Establish a Reference Center for Innovation, Assessment and Transfer (CRefIX, Centre de référence, d’innovation, d’expertise et de transfert) to foster the technological developments and information technology required for implementation of the pathway
- Measure 5: Overcome the technological, clinical and regulatory obstacles encountered along the path with respect to the three broad groups of disease concerned
- Measure 6: Establish a system to assess and validate new indications for access to genomic diagnosis
- Measure 7: Foster new skills and personnel capable of meeting the challenges of how to analyze and interpret the data
- Measure 8: Integrate ethical aspects related to the collection, storage and processing of clinical and genomic data and guarantee safe, high-quality care pathway
Establish monitoring and steering tools to make the adjustments required throughout implementation of the Plan while ensuring public involvement (Target 3)
- Measure 9: Mobilize industrial entities implicated in the project to meet technological and industrial needs at the various steps in the care pathway and promote the emergence of a "genomic medicine" channel
- Measure 10: Orientate the activities of all those involved to address industrial issues arising along the genomic care pathway
- Measure 11: Monitor developments in the field of genomic medicine at the international level
- Measure 12: Establish a research program dedicated to economic aspects of the Plan
- Measure 13: Organize the information, consultation and involvement of everyone in society who is concerned
- Measure 14: Set up the governance of the Genomic Medicine Plan
The MULTIPLI Program
Setting up a high-throughput sequencing capability across France raises a number of technological and scientific challenges. One of them is to reveal unexpected obstacles and constraints and develop appropriate solutions to the implementation of the genomic care pathway in clinical routine.
The measure #5 of the FGM 2025 Plan aims to test how the care pathway is working in situ, through the setting up of four pilot projects on cancer, rare diseases, common diseases and a sample of the general population. The MULTIPLI Program constitutes the pilot project on cancer.
Therefore, the first mission of the MULTIPLI Program is to estimate the feasibility of NGS in real settings, in order to give an answer to the measure #5 of the FMG 2025 Plan: "To detect and overcome technological, clinical and regulatory obstacles along the genomic pathway in clinical routine care".
MULTISARC Design and Objectives
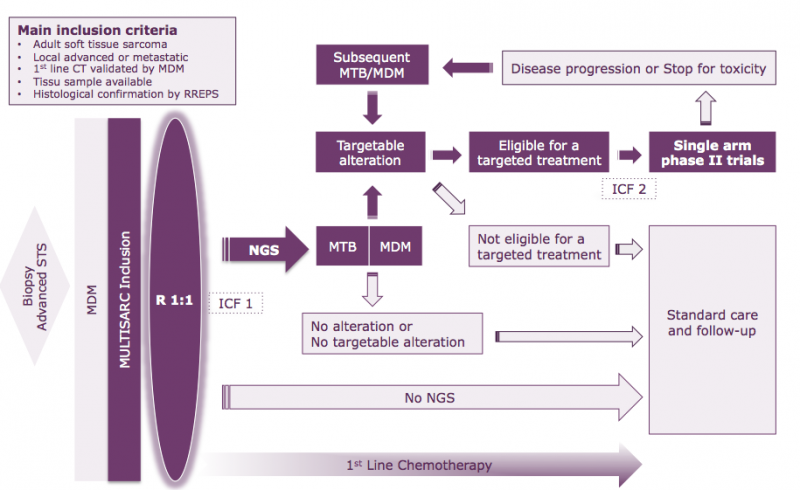
The MULTISARC trial is a multicenter clinical trial launched in January 2019. It encompasses an innovative trial driven in patients suffering from of a soft-tissue sarcoma (STS), which is a rare but high risk cancer.

The MULTISARC trial has as primary objective to assess the feasibility of high-throughput sequencing (NGS) of tumor genomes in the time frame required for the first line (induction) patient chemotherapy. The MULTISARC Primary Objective is assessed in the NGS arm and defined as the proportion of patients for whom:
- Genomic results from NGS are interpretable AND
- A validated report of exome sequencing including a MTB clinical recommendation is available within 7 weeks after the sample reception by a MULTIPLI molecular platform.
As secondary objectives, MULTISARC trial will evaluate the clinical efficacy of innovating molecules targeted on tumor genomic alterations identified during the NGS step. These MULTIPLI drugs are available thanks to a tight partnership between the academic sponsor of the Program and several pharmaceutical companies. MULTISARC trial will explore:
- Comparative efficacy of the 2 treatment strategies: "NGS" (Treatment Based on genomic results) vs "No NGS" (StandardTreatment), assessed in all randomized patients;
- Safety profile and efficacy of MULTISARC targeted treatments, assessed in the NGS arm;
- Proportion of patients with one or more alteration targetable in MULTISARC, assessed in the NGS arm.
MULTISARC Design